Calcium/calmodulin-dependent protein kinase 2 (CAMK2) belongs to the most abundant proteins in the brain, accounting for >1% of total protein. The CAMK2 family consists of four different highly similar enzymes (CAMK2A, CAMK2B, CAMK2D and CAMK2G), of which CAMK2A and CAMK2B are the most abundant proteins in the brain. Recently we identified patients carrying mutations in one of the CAMK2 genes (Küry, Am J Hum Genet 2017; Akita, Ann Clin Transl Neurol, 2018; Chi, eLife, 2018; Rizzi Am J Med Genet, 2020). Regardless which CAMK2 isozyme is affected, all reported affected individuals suffered from mild to severe intellectual disability. In addition, most patients showed impaired motor coordination, speech and language disorder and behavioral problems. In some cases epilepsy was reported.
CAMK2-related disorder is a very rare disorder. Currently we have identified 70 patients worldwide, of which the majority carry mutations in the CAMK2A gene. Functional analysis in our lab has shown that the mutations can have a Loss of Function (LoF), a Gain of Function (GoF) or a dominant negative effect on protein function, and either of these can result in a neurodevelopmental disorder.
In mice, CAMK2 was the first gene that was identified to be critical for learning and memory formation. In the lab, we have worked for more than 20 years studying the role of CAMK2 in the brain. We have mouse models for CAMK2A, CAMK2B and CAMK2G. Using these mouse models, our lab aims to investigate the function of CAMK2 in neurodevelopment and its role in neurodevelopmental disorders, using a multidisciplinary approach of molecular biology, electrophysiology and behavior.
Role and novel substrates of CAMK2 in the brain.
CAMK2 is a kinase, which means that it can phosphorylate (place a phosphate group on) other proteins (called ‘target’). Through this phosphorylation several processes in the cell are either activated or silenced. To understand which are the critical proteins regulated by CAMK2 we have deleted the CAMK2A and CAM2B gene in mice, and performed an extensive analysis to identify the most critical substrates of CAMK2 (Kool, J Neurosci, 2019).
Role of CAMK2A and CAMK2B in neurodevelopment.
CAMK2 related disorder is a neurodevelopmental disorder. However, most of the research performed on CAMK2 is done with adult animals and on the adult brain. We have generated different mouse models where we can turn the CAMK2A gene on or off at specific timepoints and even in specific brain areas, and use these mice to study the role of CAMK2 in the brain (Achterberg, , J Neurosci, 2014; Kool, Sci Rep, 2016).
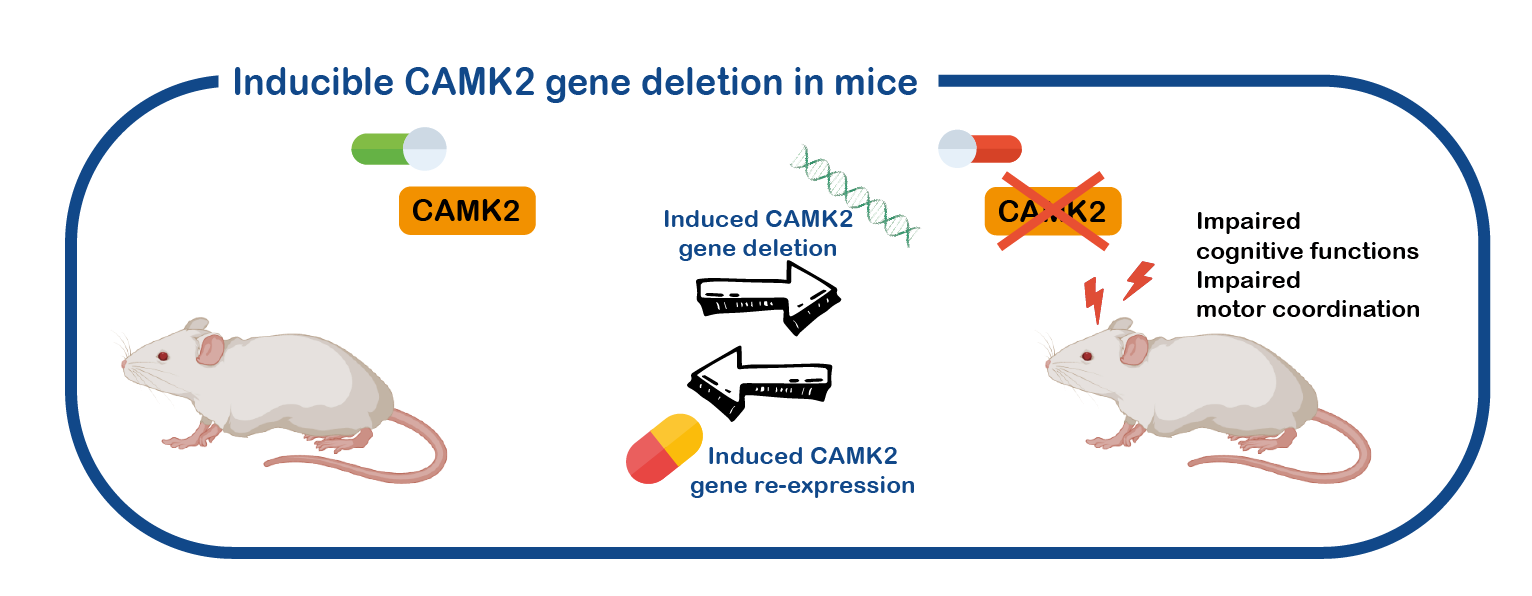
Using these mouse models, we are currently investigating the role of CAMK2 in brain development. We also use these mice to test the reversibility of the deficits, and to identify drugs that can improve the deficits.
Role of CAMK2G and CAMK2D in neurodevelopment.
Recently, we identified 2 unrelated individuals with neurodevelopmental delay carrying a mutation in the CAMK2G gene (CAMK2Gp.R292P; (Proietti Onori, Hum Mutat, 2018)). We now make use of a CAMK2G mouse model, where the gene is turned off during development but can be switched on at any desired time, allowing us to assess the neurobehavioral phenotypes, including their reversibility over time. Having identified now also patients carrying mutations in CAMK2D, a similar research strategy is planned for CAMK2D. These experiments are a first step in understanding the role of the different CAMK2s in neurodevelopment
Measuring from human neurons
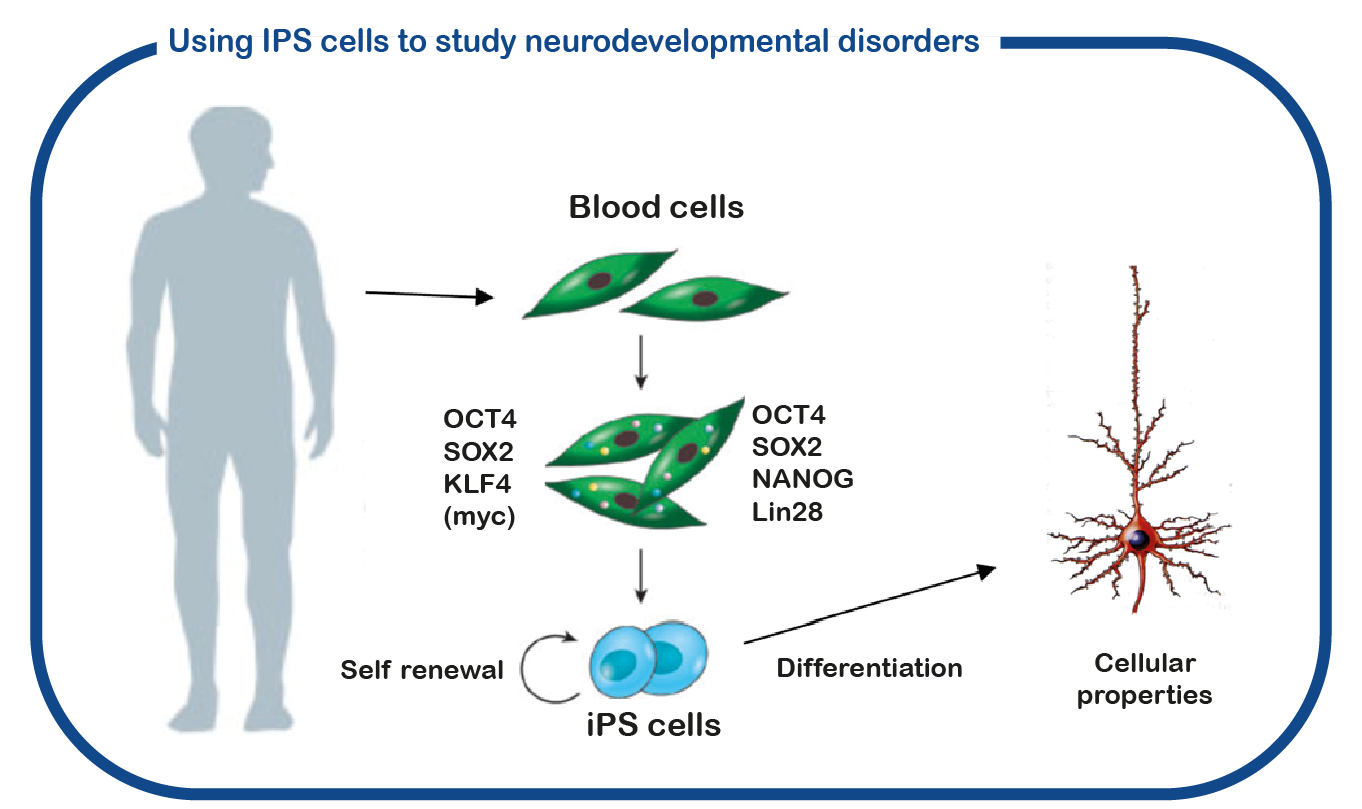
In addition to using mouse models, we also utilize induced pluripotent stem (iPS) cells. These stem cells are generated from reprogrammed blood cells donated by patients and unaffected family members. The major benefit of such iPS cells is that we can differentiate these cells into human neurons, which enables us to study human (patient) neurons in a dish. With this approach we hope to decipher how CAMK2 mutations cause neuronal dysfunction and identify novel drugs to correct neuronal function.
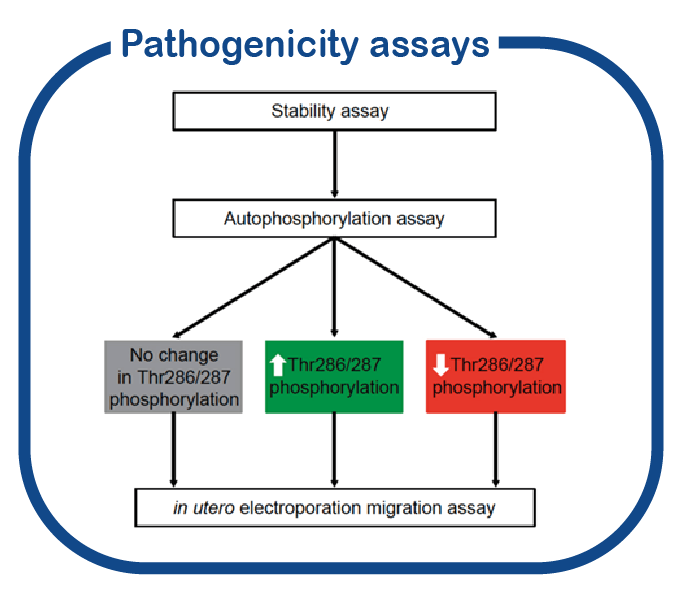
Understanding the effect of novel variants in the CAMK2 genes.
Many new variants are currently being identified in the CAMK2 genes. For several of these variants the effect (if any) on protein function is unknown. Making use of the functional genomics screen we have been developing in the last years, PRiSM (Pipeline of Rapid in silico, in vivo and in vitro Screening of Mutations; functional genomics.nl, we created a platform for functional genomics studies to assess the pathogenicity of mutations identified in CAMK2 and its downstream effectors in individuals with a neurodevelopmental disorder.
CAMK2A consists of four distinct domains: a catalytic domain containing the active-site required for CAMK2 kinase activity, a regulatory domain comprising the calcium-calmodulin binding site and the autoinhibitory domain including the Thr286 phosphorylation site. This specific structure is of crucial importance for its role as a memory molecule. When neurons communicate with each other, calcium enters into the cell. This calcium signal is very brief, but will be translated in a long-lasting signal through the activation and auto-phosphorylation of CAMK2: calcium binds CAMK2 and this binding results in a conformational change of CAMK2. This releases the kinase domain from the regulatory domain and allows for a process called autophosphorylation, where an active CAMK2 subunit can place a phosphate group on the now available T286 residue of CAMK2. When the calcium levels go down again in the cell, calcium is released from CAMK2 and CAMK2 will maintain its open and active state due to the phosphate group placed on the T286 residue. This process is essential for memory formation.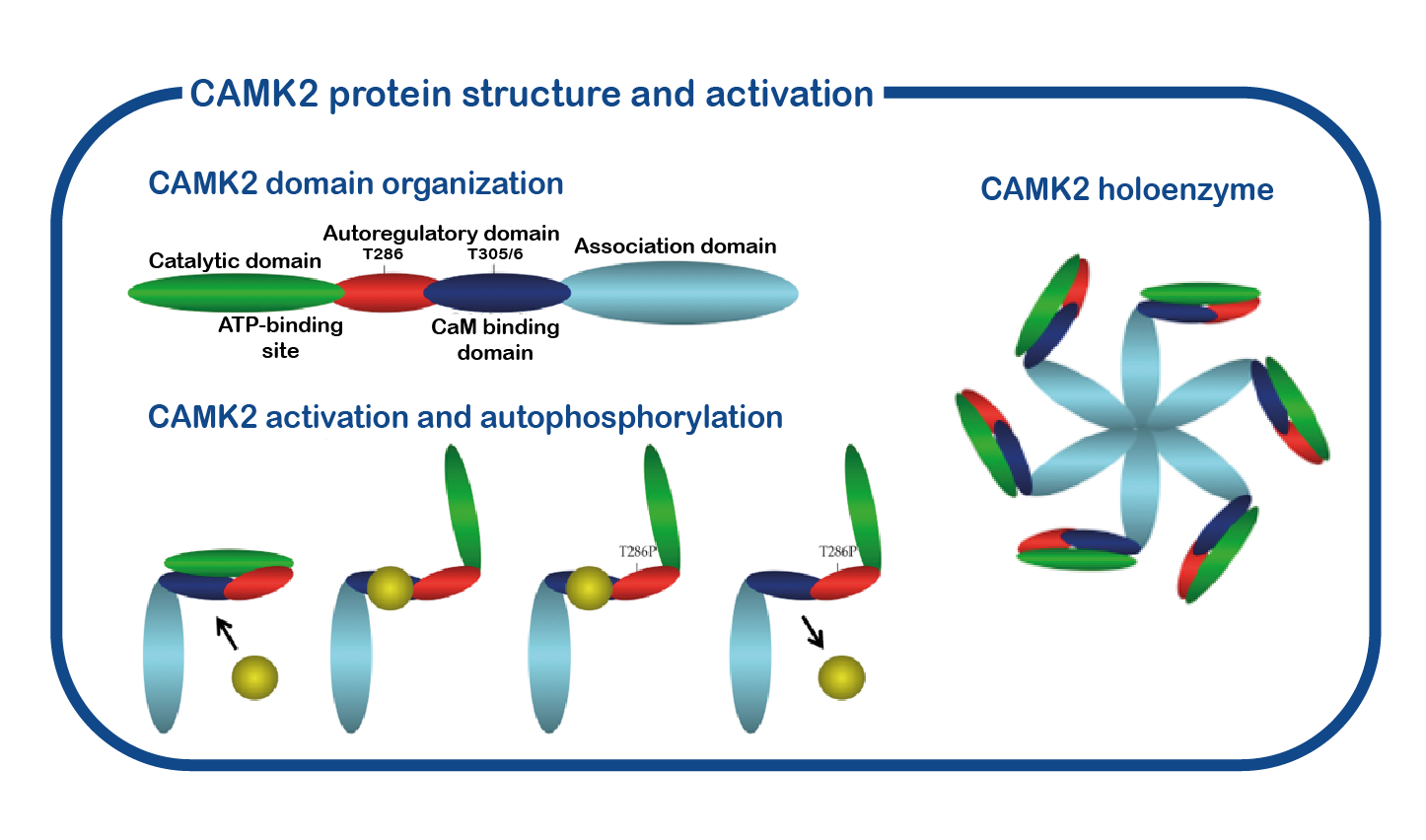
Rigter PMF, et.al. (2024) Role of CAMK2D in neurodevelopment and associated conditions. Am J Hum Genet. Pubmed
Rigter PMF, de Konink C, van Woerden GM. (2023). Loss of CAMK2G affects intrinsic and motor behavior but has minimal impact on cognitive behavior. Front Neurosci. Jan 6;16:1086994. Pubmed
Rigter PMF, et.al. (2022) Adult Camk2a gene reinstatement restores the learning and plasticity deficits of Camk2a knockout mice. Pubmed
Dwyer BK, et.al. (2022) Case Report: Developmental Delay and Acute Neuropsychiatric Episodes Associated With a de novo Mutation in the CAMK2B Gene (c.328G>A p.Glu110Lys) Front Pharmacol. 10;13:794008 Pubmed
Onori MP & van Woerden GM. (2021) Role of calcium/calmodulin-dependent kinase 2 in neurodevelopmental disorders. Brain Res Bull. S0361-9230(21)00090-3 Pubmed
Moro A, et.al. (2020) CaMKll controls neuromodulation via neuropeptide gene expression and axonal targeting of neuropeptide vesicles. PLoS Biol. Pubmed
Kool MJ, et al. (2019) CAMK2-dependent signaling in neurons is essential for survival. J Neurosci. 39; 5424–39. Pubmed
Vargas JY, et al. (2019) The Wnt/Ca 2+ pathway is involved in interneuronal communication mediated by tunneling nanotubes. EMBO J. Pubmed
Onori MP, et al. (2018) The intellectual disability-associated CAMK2G p.Arg292Pro mutation acts as a pathogenic gain-of-function. Hum Mutat. 39; 2008–24. Pubmed
Küry S, et al. (2017) De Novo Mutations in Protein Kinase Genes CAMK2A and CAMK2B Cause Intellectual Disability. Am J Hum Genet. 101; 768–88. Pubmed
Kool MJ, et.al. (2016) The molecular, temporal and region-specific requirements of the beta isoform of Calcium/Calmodulin-dependent protein kinase type 2 (CAMK2B) in mouse locomotion. Sci Rep. Pubmed
Achterberg KG, et al. (2014) Temporal and region-specific requirements of αCaMKII in spatial and contextual learning. J Neurosci. 34; 11180–7. Pubmed
Woerden G, et.al. (2009) betaCaMKll controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 12(7); 823-5. Pubmed
Hojjati M, et.al. (2007) Kinase activity is not required for alphaCaMKll-dependent presynaptic plasticity at CA3-CA1 synapses. Nat Neurosci. 10(9); 1125-7. Pubmed
Hansel C, et.al. (2006) alphaCaMKll is essential for cerebellar LTD and motor learning. Neuron. 51(6); 835-43. Pubmed
Elgersma Y, et.al. (2004) Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase ll function in plasticity and cognition. J Neurosci. 24(39); 8410-5. Pubmed
Elgersma Y, et.al. (2002) Inhibitory autophosphorylation of CaMKll controls PSD association, plasticity and learning. Neuron. 36(3); 493-505. Pubmed
