Costello and CFC are autosomal dominant genetic disorders. These disorders belong to a large group of disorders referred to as RASopathies for which the gene products (proteins) have related functions. These proteins are forming a signaling cascade in the cell. The central nodes of this signaling pathway are formed by the RAS proteins. The most common mutation affecting this pathway is the NF1 mutation. The RAS pathway (depicted in red in the figure) is closely connected to the mTOR pathway, and together they control protein synthesis in the cell. In dividing cells, RAS signaling is necessary for making new proteins to promote growth, and increased RAS signaling in these cells may lead to cancer. In the brain, RAS signaling is needed to synthesize proteins to form new synaptic connections between neurons. Enhanced RAS signaling in the brain leads to cognitive impairments.
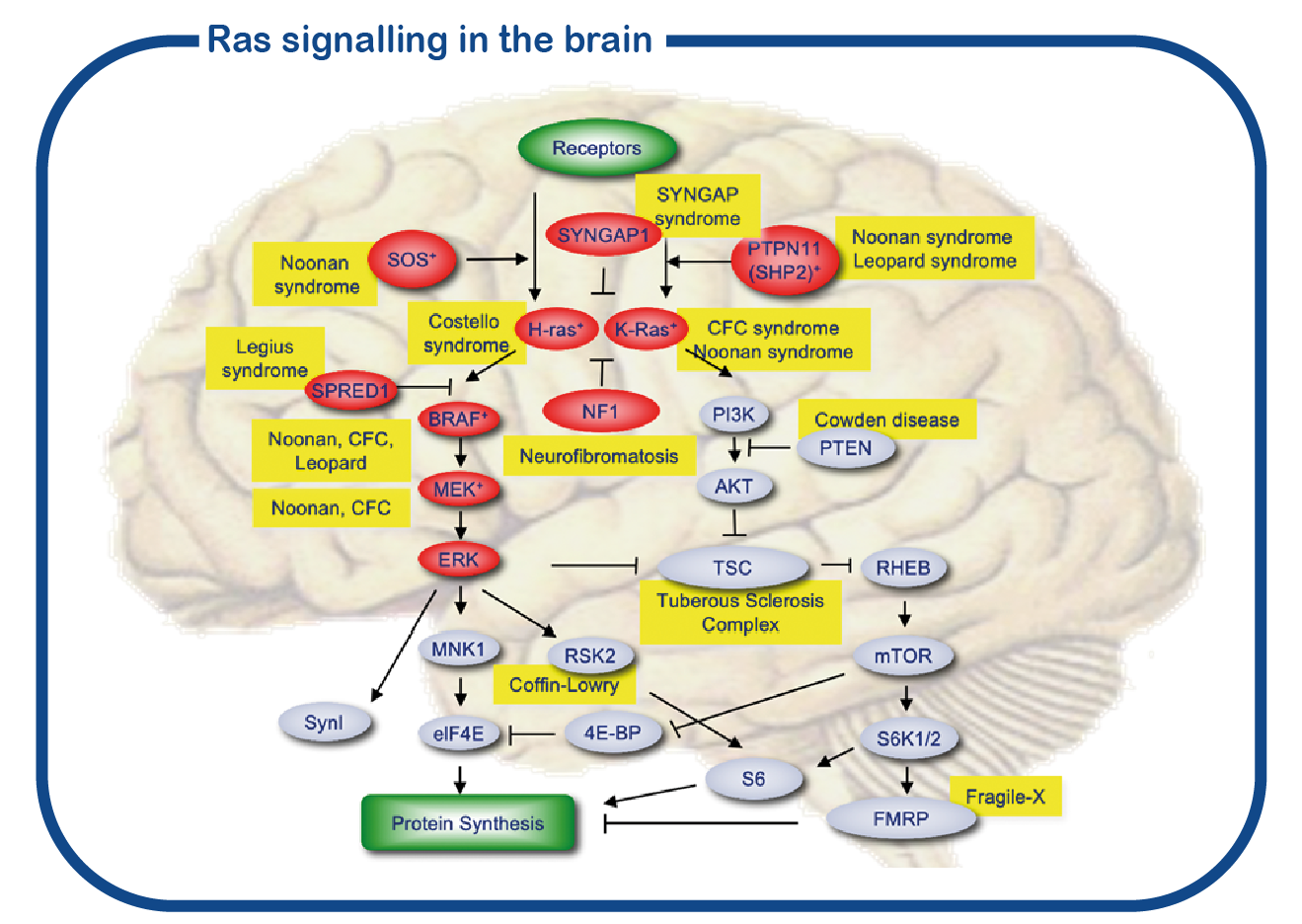
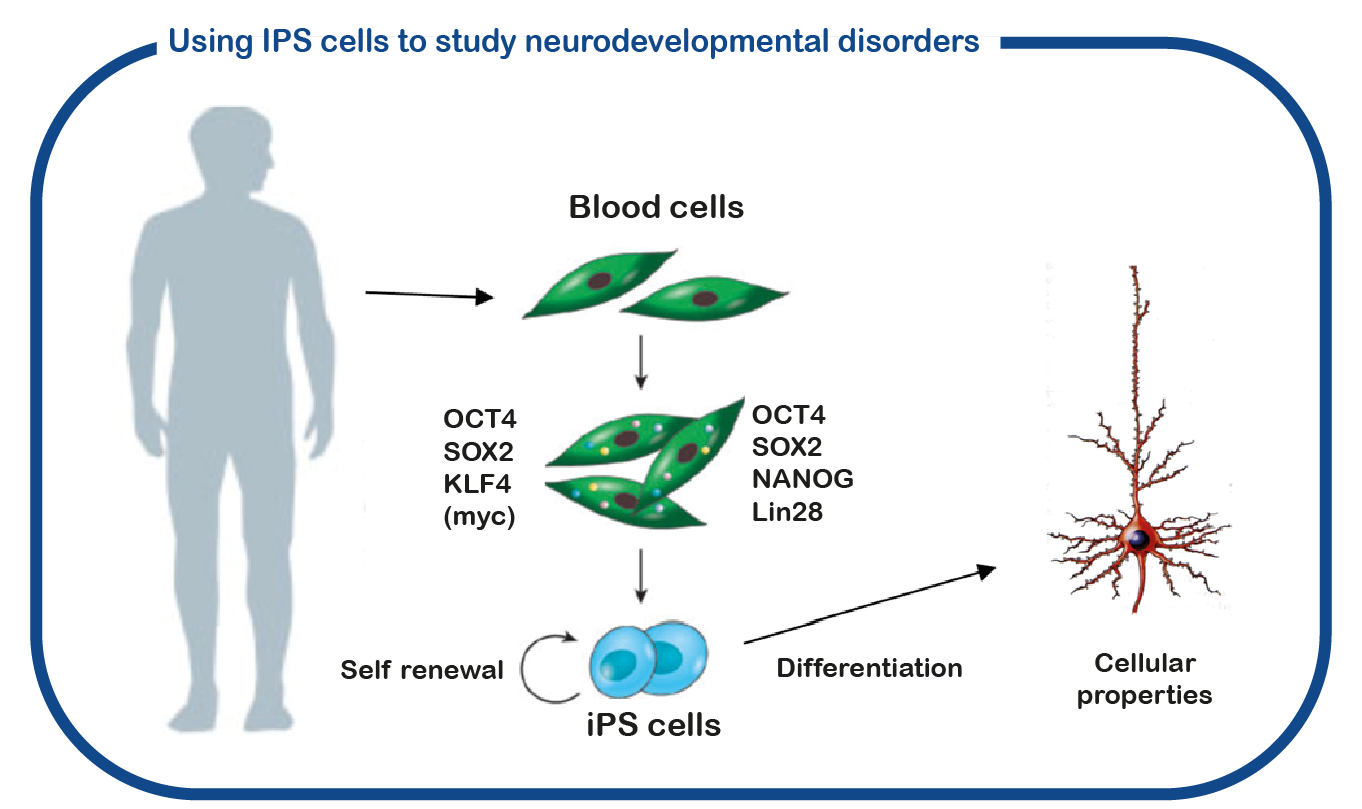
At ENCORE we mostly study the RASopathies by studying NF1 and Costello syndrome. We primarily focus on the cognitive deficits that are associated with increased activity of the RAS signaling pathway in RASopathies. For that we make use of the Nf1 and Costello mouse model (Omrani, Molecular Psychiatry, 2015; Schreiber, Scientific reports, 2017). We use cognitive tests to assess the learning deficits and to try to improve these deficits with drugs that target the RAS pathway. In addition to using mouse models, we also utilize induced pluripotent stem (iPS) cells. These stem cells are generated from reprogrammed blood cells donated by patients and unaffected family members. The major benefit of such iPS cells is that we can differentiate these cells into human neurons, which enables us to study human (patient) neurons in a dish. With this approach we hope to decipher how impaired RAS signaling causes neuronal dysfunction and identify novel drugs to correct neuronal function.
Gripp KW, et al. (2019) Costello Syndrome: Clinical phenotype, genotype, and management guidelines. Am J Med Gent A. 179 (9); 1725-1744. Pubmed
Schreiber J, et al. (2017) Mechanisms underlying cognitive deficits in a mouse model for Costello Syndrome are distinct from other RASopathy mouse models. Sci Rep. Pubmed
Wang T, et.al. (2015) In vivo synaptic transmission and morphology in mouse models of tuberous sclerosis, Fragile X syndrome, Neurofibromatosis type 1, and Costello syndrome. Front Cell Neurosci. Pubmed
Omrani A, et al. (2015) HCN channels are a novel therapeutic target for cognitive dysfunction in Neurofibromatosis type 1. Mol Psychiatry 20; 1311–21. Pubmed
Beukers, W. et al. (2013) HRAS mutations in bladder cancer at an early age and the possible association with the Costello Syndrome. Eur J Hum Genet DOI: 10.1038/ejhg.2013.251. Pubmed
Krab, L.C. et al. (2008) Oncogenes on my mind: ERK and MTOR signaling in cognitive diseases. Trends Genet 24, 498–510. Pubmed
Kushner, S.A. et al. (2005) Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci 25, 9721–9734. Pubmed
