Neurofibromatosis type 1 (NF1) is an autosomal dominant genetic disorder caused by a heterozygous loss-of-function mutation in the NF1 gene. NF1 has a birth incidence of approximately 1:2000. The partial inactivation of the NF1 gene can cause variable symptoms affecting skin, bone and nervous system and NF1 is also associated with an increased risk of benign and malignant tumor formation. NF1 is frequently associated with cognitive disabilities. These cognitive deficits include deficits in attention, visual-spatial abilities, motor learning, executive functioning, and intelligence (Krab, The Journal of Pediatrics, 2009, 2008).
NF1 cognitive research.
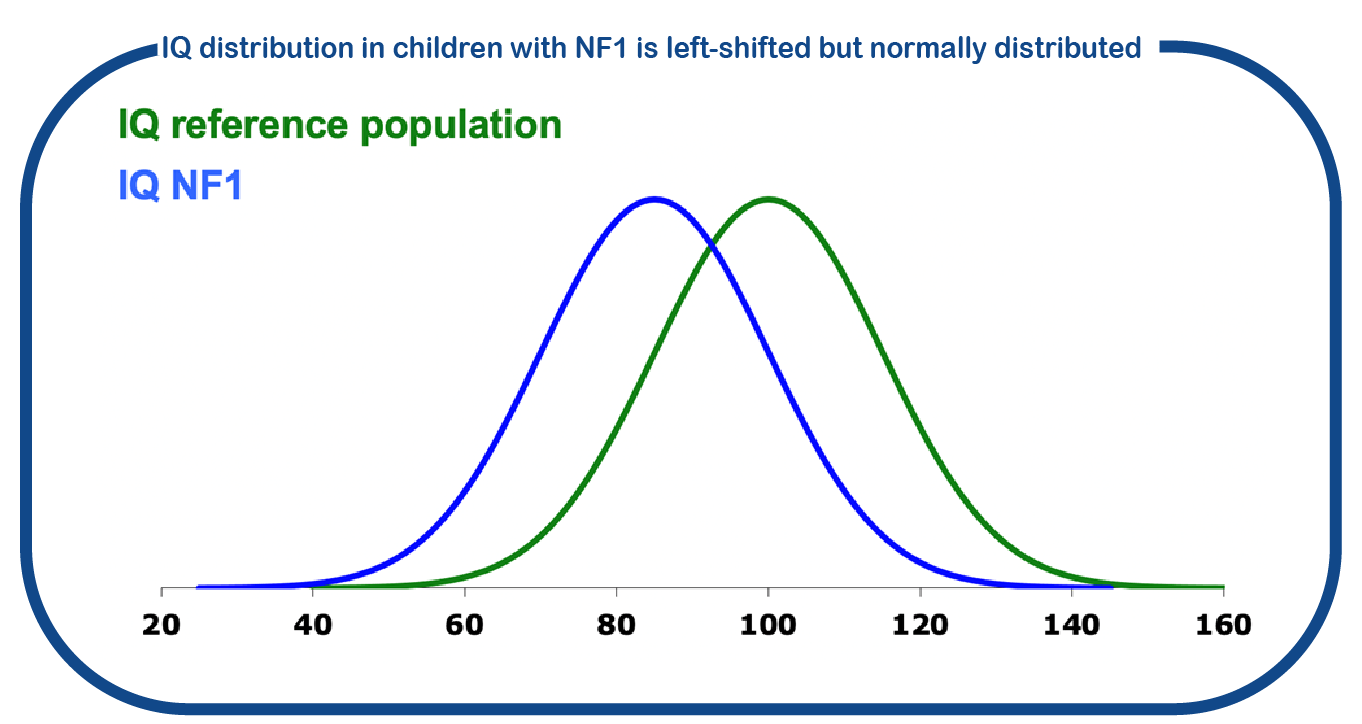
The ENCORE NF1 expertise center at Erasmus MC has a long history of research into the cognitive problems associated with NF1. Recently, in a study with 500 children we showed that although the mean full-scale intelligence quotient of children with NF1 is shifted to the left (mean IQ 88), the variability in cognitive ability is similar to the general population (Ottenhoff, Genet Med, 2020). This indicates that the presence of the NF1 mutation is affecting IQ with about 12 points, regardless the type of mutation in NF1. Only in NF1 individuals with a chromosomal deletion, this shift is significantly larger. This also indicates that there is no significant role of so called ‘modifier genes’ that can potentially change the effect of NF1 on brain function.
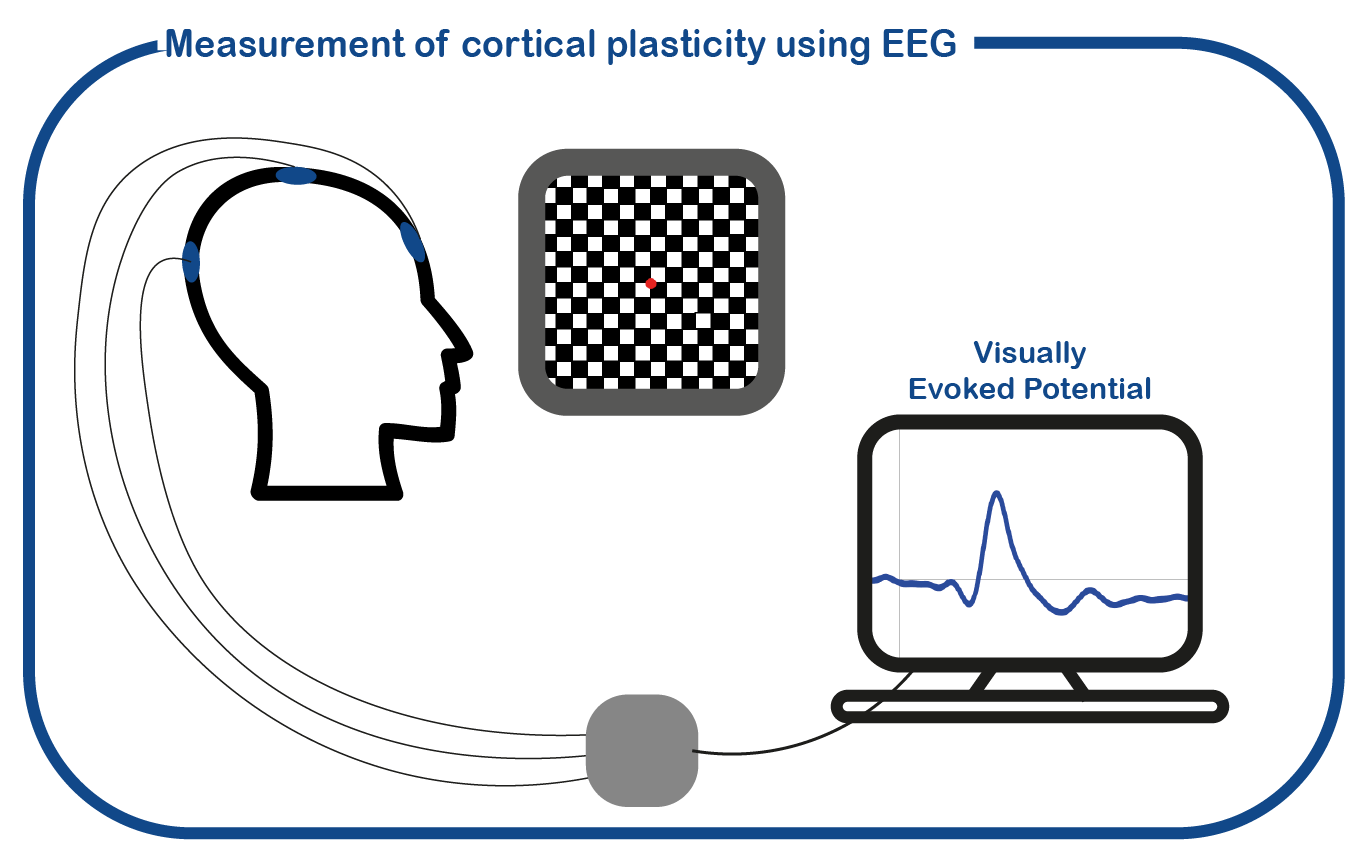
Studies of the cellular mechanism underlying the cognitive deficits associated with NF1 have largely focused on animal models of NF1. Based on these studies, the hypothesized cause of cognitive disabilities results from increased activity of inhibitory interneurons that decreases synaptic plasticity. Whether changes in neuronal plasticity are also underlying the cognitive deficits in NF1 patients is unknown. To investigate the role of cortical plasticity in the cognitive deficits in adults with NF1, we use non-invasive neurophysiological measures as transcranial magnetic stimulation (TMS) and electroencephalography (EEG).
Despite several clinical trials aimed at improving cognitive deficits in NF1, we have not been able to identify an effective treatment yet (Krab, The Journal of the American Medical Association, 2008). Statins improved neuronal plasticity and learning deficits in a Nf1 mouse model, however, it had no effect on cognitive function, attention and behavior in NF1 children (Van de Vaart, The Lancet Neurology, 2013). Furthermore, the ENCORE-laboratory has shown that the cognitive deficits in Nf1 mice are caused by impaired function of HCN1-channels (hyperpolarization-activated cyclic nucleotide-gated channels) (Omrani, Molecular Psychiatry, 2015). An agonist (stimulator) of the HCN1 channel, lamotrigine, could rescue deficits in inhibition and plasticity in animal models of NF1. Whether this is also the case in human, is currently being investigated by means of a randomized controlled trial.
Ongoing clinical studies.
VEP-NF1 study: to investigate plasticity in the visual cortex in NF1 patients, we measure visual evoked potentials (VEPs) in response to visual stimulation. Additionally, to study the ability and efficiency of the visual system to perform actions, we assess eye- and hand-tracking measures during specific coordination tasks
NF1 Tumor-related research.
NF1 patients are predisposed to develop cancer. Almost all individuals with NF1 present with benign, dermal neurofibromas associated with peripheral nerves. Many also develop deeper-seated plexiform neurofibromas that may transform, through a distinct intermediary, precursor lesion atypical neurofibromas, into malignant peripheral nerve sheath tumors (MPNST). NF1 patients have life-time risk of 8 – 16% to develop MPNST which are a major cause of mortality in this patient group. MPNST are aggressive soft tissue sarcomas that metastasize easily and are therapy resistant translating into a dismal prognosis particularly for those patients confronted with unresectable or advanced disease. In the department of Medical oncology, Erasmus MC, the determining factors and molecular drivers underlying the malignant transformation of PNF into MPNST are being studied with a particular interest for microRNAs. We demonstrated that deregulated microRNAs in MPNST contribute to cancer-related processes such the ability to invade surrounding tissue and to metastasize (Amirnasr, Scientific Reports, 2020). A better understanding of the precise involvement of microRNAs in MPNST – and atypical neurofibromas and plexiform neurofibromas – biology may yield novel, more effective, therapeutic strategies. Another research goal is to identify biomarkers e.g. microRNAs in the NF1 patient’s circulation that signal the development of MPNST enabling clinical intervention at an early stage.
Dhaenens BAE, et.al. (2024) The PlexiQoL, a patient-reported outcome measure on quality of life in neurofibromatosis type 1-associated plexiform neurofibroma: translation, cultural adaptation and validation into the Dutch language for the Netherlands. J Patient Rep Outcomes. Pubmed
Bennebroek CA, et.al. (2023) Treatment evaluation by volumetric segmentation in pediatric optic pathway glioma: evaluation of the effect of bevacizumab on intra-tumor components. J Neurooncol. Pubmed
Taal W, et.al. (2023) Symptomatische tumoren bij neurofibromatose type 1 Symptomatic tumors in neurofibromatosis type 1: a diagnostic challenge. Ned Tijdschr Geneeskd. Pubmed
Dhaenens BAE, et.al. (2023) Health-related quality of life of children with neurofibromatosis type 1: Analysis of proxy-rated PedsQL and CHQ questionnaires. Eur J Paediatr Neurol. Pubmed
Carton C, et.al. (2023) ERN GENTURIS NF1 Tumour Management Guideline Group. ERN GENTURIS tumour surveillance guidelines for individuals with neurofibromatosis type 1. Pubmed
Castricum J, et.al. (2023) Visual-spatial and visuomotor functioning in adults with neurofibromatosis type 1. J Intellect Disabil Res. Pubmed
Ottenhoff MJ, et.al. (2022) Cerebellum-dependent associative learning is not impaired in a mouse model of neurofibromatosis type 1. Sci Rep. Nov 9;12(1):19041. Pubmed
Douben HCW, et.al. (2022) High-yield identification of pathogenic NF1 variants in skin fibroblast transcriptome screening after apparently normal diagnostic DNA testing. Hum Mutat. Pubmed
Castricum J, et.al. (2022) Plasticity of visual evoked potentials in patients with neurofibromatosis type 1. Clin Neurophysiol. Pubmed
Lubbers K, et.al. (2022) Autism Symptoms in Children and Young Adults With Fragile X Syndrome, Angelman Syndrome, Tuberous Sclerosis Complex, and Neurofibromatosis Type 1: A Cross-Syndrome Comparison. Front Psychiatry. Pubmed
Dhaenens BAE, et.al. (2021) Lessons learned from drug trials in neurofibromatosis: A systematic review. Eur J Med Genet. 2021 Jul 5:104281. Pubmed
Castricum J, Tulen JHM, Taal W, Rietman AB, Elgersma Y (2021). Attention and Motor Learning in Adult Patients with Neurofibromatosis Type 1. J Atten Disord. Pubmed
Dhaenens BAE, et.al. (2021). Identifying challenges in neurofibromatosis: a modified Delphi procedure. Eur J Hum Genet. 26:1–9 Pubmed
Fangusaro J, et.al. (2020) Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 21(6); e305-16. Pubmed
Amirnasr A, et.al. (2020) Deregulated microRNAs in neurofibromatosis type 1 derived malignant peripheral nerve sheet tumors. Sci. Rep. 10:2927. Pubmed
Castricum J, et.al. (2020) Motor cortical excitability and plasticity in patients with neurofibromatosis type 1. Clin Neurophysiol. 131(11); 2673-81. Pubmed
Ottenhoff MJ, et al.(2020) Examination of the genetic factors underlying the cognitive variability associated with neurofibromatosis type 1. Genet Med.1-9. Pubmed
Frebourg T, et.al. (2020) Guidelines for the Li-Fraumeni and heritable TP53 related cancer syndromes. Eur J Hum Genet. 28(10); 1379-86. Pubmed
Siebelt M, et al. (2019) Congenital Forearm Pseudarthrosis, a Systematic Review for a Treatment Algorithm on a Rare Condition. J Pediatr Orthop. 40; e367-74. Pubmed
Vos JR, et.al. (2019) Boosting care and knowledge about hereditary cancer: European Reference Network on Genetic Tumour Risk Syndromes. Fam Cancer. 18(2); 281-4. Pubmed
Koczkowska M, et.al. (2019) Expanding the clinical phenotype of individuals with a 3-bp in-frame deletion of the NF1 gene (c.2970_2972del): an update of genotype-phenotype correlation. Genet. Med. 21(4); 867-76. Pubmed
Rietman AB, et al. (2018) Emotional and behavioral problems in children and adolescents with neurofibromatosis type 1. Am J Med Genet B Neuropsychiatr Genet. 177; 319–28. Pubmed
Rietman AB, et al. (2018) Worries and needs of adults and parents of adults with neurofibromatosis type 1. Am J Med Genet A. 176; 1150–60. Pubmed
Eijk S, et al. (2018) Autism Spectrum Disorder in an Unselected Cohort of Children with Neurofibromatosis Type 1 (NF1). J Autism Dev Disord. 48; 2278–85. Pubmed
Amirnasr A, et.al. (2017) Expression and inhibition of BRD4, EZH2 and TOP2A in neurofibromas and malignant peripheral nerve sheath tumors. PLoS One. 12(8); e0183155. Pubmed
Rietman AB, et al. (2017) Development of emotional and behavioral problems in neurofibromatosis type 1 during young childhood. Am J Med Genet A. 173; 2373–80. Pubmed
Rietman AB, et al. (2017) Motor problems in children with neurofibromatosis type 1. J Neurodev Disord. Pubmed
van der Vaart T, et al. (2016) Behavioral and cognitive outcomes for clinical trials in children with neurofibromatosis type 1. Neurology 86;154–60. Pubmed
Omrani A, et al. (2015) HCN channels are a novel therapeutic target for cognitive dysfunction in Neurofibromatosis type 1. Mol Psychiatry 20; 1311–21. Pubmed
Omrani A, et.al. (2015) Neurofibromin regulates HCN activity in Parvalbumin-positive interneurons. Mol Psychiatry. 20; 1263. Pubmed
Van Der Vaart, T. et al. (2013) Simvastatin for cognitive deficits and behavioural problems in patients with neurofibromatosis type 1 (NF1-SIMCODA): a randomised, placebo-controlled trial. Lancet Neurol 12, 1076–1083. Pubmed
van Minkelen, R. et al. (2013) A clinical and genetic overview of 18 years neurofibromatosis type 1 molecular diagnostics in the Netherlands. Clin Genet. Pubmed
Acosta, M.T. et al. (2012) The Learning Disabilities Network (LeaDNet): using neurofibromatosis type 1 (NF1) as a paradigm for translational research. Am J Med Genet A 158, 2225–2232. Pubmed
van der Vaart, T. et al. (2011) Motor deficits in neurofibromatosis type 1 mice: the role of the cerebellum. Genes Brain Behav 10, 404–409. Pubmed
Krab, L.C. et al. (2011) Motor learning in children with neurofibromatosis type I. Cerebellum 10, 14–21. Pubmed
Krab, L.C. et al. (2009) Health-related quality of life in children with neurofibromatosis type 1: contribution of demographic factors, disease-related factors, and behavior. J Pediatr 154, 420–5, 425.e1. Pubmed
Denayer, E. et al. (2008) Spred1 is required for synaptic plasticity and hippocampus-dependent learning. J Neurosci 28, 14443–14449. Pubmed
Cui, Y. et al. (2008) Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135, 549–560. Pubmed
Krab, L.C. et al. (2008) Impact of neurofibromatosis type 1 on school performance. J Child Neurol 23, 1002–1010. Pubmed
Krab, L.C. et al. (2008) Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. JAMA 300, 287–294. Pubmed
Balgobind, B.V. et al. (2008) Leukemia-associated NF1 inactivation in patients with pediatric T-ALL and AML lacking evidence for neurofibromatosis. Blood 111, 4322–4328. Pubmed
van Engelen, S.J.P.M. et al. (2008) Quantitative differentiation between healthy and disordered brain matter in patients with neurofibromatosis type I using diffusion tensor imaging. AJNR Am J Neuroradiol 29, 816–822. Pubmed
Oostenbrink, R. et al. (2007) Parental reports of health-related quality of life in young children with neurofibromatosis type 1: influence of condition specific determinants. J Pediatr 151, 182–6– 186.e1–2. Pubmed
Cnossen, M.H. et al. (1998) Minor disease features in neurofibromatosis type 1 (NF1) and their possible value in diagnosis of NF1 in children. J Med Genet 35, 624–627. Pubmed
Cnossen, M.H. et al. (1998) A prospective 10 year follow up study of patients with neurofibromatosis type 1. Arch. Dis. Child. 78, 408–412. Pubmed
Cnossen, M.H. et al. (1997) Endocrinologic disorders and optic pathway gliomas in children with neurofibromatosis type 1. Pediatrics 100, 667–670. Pubmed
Cnossen, M.H. et al. (1997) Diagnostic delay in neurofibromatosis type 1. Eur J Pediatr 156, 482–487. Pubmed
Cnossen, M.H. et al. (1997) Deletions spanning the neurofibromatosis type 1 gene: implications for genotype-phenotype correlations in neurofibromatosis type 1? Hum Mutat 9, 458–464. Pubmed
