Rianne Oostenbrink receives Rare Angel Award
Pediatrician Rianne Oostenbrink has received the Rare Angel Award. She was presented with the award last Friday by the VSOP, on Rare Disease Day, for her efforts in the field of neurofibromatosis.
A Rare Angel Award is given to someone who has made a special contribution to patients with a rare condition. Rianne is committed to children who have the rare condition neurofibromatosis. This is a genetic condition that causes tumors on nerve tissue.
Proud
The Neurofibromatosis patient association (NFVN) nominated her for the award. According to chairwoman Ine Israëls, Rianne deserves the award because she is the driving force behind the NF1 Care Network, which consists of the Erasmus MC expertise center for neurofibromatosis type 1,eleven treatment centres and four intervention centres. In addition, it is committed to European cooperation and to the roll-out and implementation of new medication.
Israëls: ‘Rianne is a very enthusiastic and knowledgeable person. She has given NF1 much more exposure. We are proud that she has won the Rare Angel Award.’
Improve
Rianne herself is ‘very surprised and honoured’. ‘What I think is great is that the initiative comes from the patient association. That does mean something.’ According to Rianne, it reflects the collaboration between the expertise centre and the patient association. ‘We work together to improve care for patients with NF.’ She also calls this award a great incentive for the future, with a number of ongoing research projects and activities for the organisation of patient care, together with colleagues from ENCORE and the national NF1 expertise network.
Film Rare Diseases Center
Rianne can also be seen in the film of the Rare Diseases Center. Have you never seen it? Then it is definitely recommended to see it here.
Ype Elgersma in Time for MAX broadcast “Attention for epilepsy”
Last Thursday, January 23, Tijd voor MAX focused on epilepsy, a brain disorder that affects 200,000 people in the Netherlands. The guest is presenter Miljuschka Witzenhausen. She has a daughter with epilepsy and is an ambassador for EpilepsieNL. Also a guest is professor Ype Elgersma from the Erasmus MC, who conducts groundbreaking research into customized gene therapy.
Click here to watch the episode.
Epilepsy can happen to anyone, at any stage of life. Sometimes with a clear cause, such as an accident or a cerebral haemorrhage, more often without a clear cause. Some people have an epileptic seizure every day, others have it once a year. Thirty percent of people with epilepsy do not become seizure-free. These people have more than one seizure per month. This always happens unexpectedly: while shopping, doing odd jobs or on the way to work.
Presenter Miljuschka Witzenhausen asks for attention for this brain disorder.
"Epilepsy entered our lives nine years ago. It disrupts my daughter's life every day. We are still looking for answers and solutions every day," the presenter said.
Powerless and insecure In Time for MAX the story of Marije (20).
She is seven years old when she is diagnosed with epilepsy. Her childhood is determined by it: every day she suffers from absences and sometimes she has a seizure in which she loses consciousness. She often feels incredibly powerless and insecure. Her hope is placed on research to make her life more bearable.
No treatment for four-year-old Inti.
Four-year-old Inti has a genetic disorder that results in epilepsy, for which there is no treatment. She is given the highest dosage of medication, but still has twenty to forty seizures per month. That is why she has to be monitored 24 hours a day, so that she can act immediately if she has a seizure. A huge task for her parents, because someone has to stay with her at all times, even at night. All hope is placed on a customized treatment through genetic therapy.
Customized genetic therapy
Prof. Dr. Ype Elgersma is trying to develop a therapy for Inti; a treatment that should correct the genetic error. According to the professor, that is the solution: "in a few years we want to have this therapy in the clinic." The research he is doing focuses on children. "In more than thirty percent of children with epilepsy, a mutation in the DNA is the underlying cause," says Elgersma. "If you track down this genetic error, you can repair it with therapy."
Scientists throughout the Netherlands are committed to improving the lives of people with epilepsy through research every day. In the coming years, EpilepsieNL wants to focus on breakthroughs in scientific research into the treatment of epilepsy. Important spearheads are being able to predict seizures in the future and being able to cure epilepsy. Find out more about this by watching Time for Max.
New funding for CAMK2 syndrome research
The van Woerden lab has recently received funding from the Merel Foundation to test the effect of candidate variants in the Camk2 genes, found in patients with neurodevelopmental disorder.
With this money we will use the PRiSM screen to determine whether the variants are indeed harmful and in what way. This information is crucial for the genotype-phenotype correlation analysis, which is important to ultimately determine prognoses for newly found mutations. In addition, this helps with the direct diagnosis of the patient and the possible treatment options. The van Woerden is very pleased with this award.
The de Merel foundation aims to promote health care in the broadest sense of the word, including by coordinating and stimulating scientific research in the field of health care.
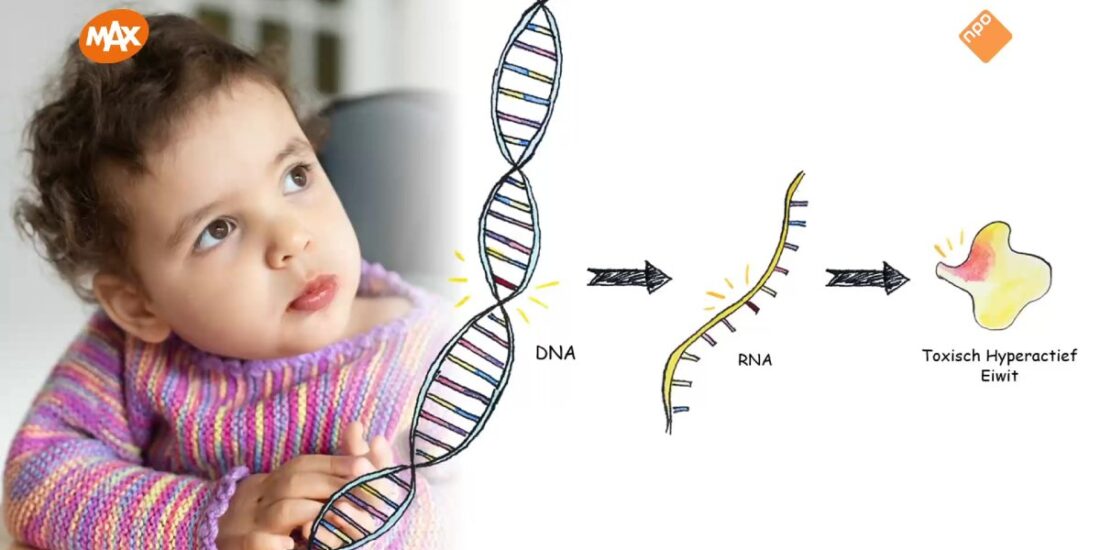
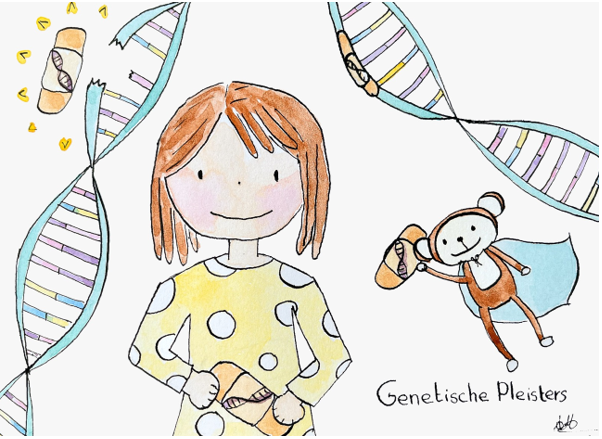
The Sophia Lichtjesdiner Lights the Way to Genetic Patches
On Saturday 7 December, the ninth edition of the Sophia Lichtjesdiner took place, a benefit gala organised for and by the “Friends of Sophia”. This year, our department had the unique opportunity to present one of our research lines during the auction.
During a spectacular evening in the Laurenskerk, Ype Elgersma told the 400-strong audience about the “genetic patches” that he, together with Annelot van Esbroeck and their research team, wants to develop. With an efficient screening pipeline, they hope to develop personalized antisense oligonucleotides (ASOs, a form of RNA therapy) to treat ultra-rare developmental disorders. After a pitch of only two minutes, the auctioneer encouraged the audience to show their support by raising their hands. “As many hands as possible in the air for 25,000 euros per hand”, after which dozens of hands went up in the air.
This year, the Lichtjesdiner reached a record amount of 1.7 million euros! Of this amount, an impressive 500,000 euros has been reserved for the development of the “genetic patches” by Ype and Annelot. Thanks to this support, they can further develop their pipeline for personalized ASOs in the coming years. “This is not only support for research into innovative therapies, but also hope for parents and children for whom no other options are currently available,” says Ype.
ENCORE receives €60.000 from the vASN for Angelman syndrome research
On November 23, 2024, the information day of the Association Angelman Syndrome Netherlands (vASN) took place. The members, donors and relations raised a large amount through fundraising campaigns and donations.
The ENCORE expertise centre has been presented with a cheque for €60,000 for the implementation of scientific research into Angelman syndrome.
During the annual information day, the latest developments in the field of Angelman syndrome are discussed, both in the field of (scientific) research and in the field of support in daily life.
For more information about the association, please visit https://www.angelmansyndroom.nl/.
Kick-off for SYNGAP1 study in adults!
Participate in our research and contribute to medical knowledge about SYNGAP1 syndrome!
Continue ReadingThe CAMK2 Outcome Measures Study has officially started!
The CAMK2 Outcome Measures Study has officially started!
This month, the first two CAMK2 families traveled all the way from Italy to the Erasmus MC to participate in our efforts to identify feasible outcome measures for future intervention trials.
As a proof-of-concept, we are focusing on patients with two specific gain-of-function mutations in the CAMK2A and beta isoform. During their stay, the families visited our Child Brain Lab and were seen by our multi-disciplinary CAMK2 expert team which consists of a child psychiatry team, speech-language therapist, physical therapists, pediatric neurologist, and developmental & genetic pediatrician.
We are grateful to the families for participating in this study and look forward to meeting more members of the CAMK2 community in the coming years.
This project is funded by the Dutch Brain Foundation.
Aware of patients with a CAMK2B (Pro139Leu) or CAMK2A (Thr286XX) disorder? Please contact our team: camk2disorders@erasmusmc.nl.
ASA conference Coventry 2024
On August 1 and 2, 2024, several members of the ENCORE Expertise Center for AS attended the ASA Scientific Conference, this time held in Coventry, UK.
It was an inspiring meeting with reunions of familiar faces and interesting presentations. Edwin Mientjes, Doesjka Hagenaar, Karen Bindels-de Heus and Marie-Claire de Wit also gave a presentation on their research. Karen was surprised by the ASA board with a Certificate of Gratitude for her recent promotion. We also had a useful meeting with our partners from the growing European network for AS.


Tour de Bunzl cycles for the Angelman Association
On September 15, 2024, a huge amount of money was raised for the Angelman association during the Tour de Bunzl: €40,818!
Bunzl Nederland organizes this annual cycling tour through the hills of Valkenburg. A group of employees of one of the Bunzl companies cycle along. The sponsored money is generated by various suppliers who donate a lot of money to a good cause. This year, the Angelman Syndrome was chosen. The colleagues of Angel Saar's mother, who works at King - Bunzl in Tiel, also participated this year for this fantastic cause. At the end of the ride, the check was presented to the association under the watchful eye of the chairman, the treasurer, Angel Saar and Saar's mother.
Hora est! Promotion Karen Bindels-de Heus
On July 3, 2024, pediatrician-EAA Karen Bindels-de Heus successfully defended her thesis “Angelman Syndrome in Children”.
In several studies she investigated the natural course of Angelman Syndrome (AS) in children with specific attention to development, epilepsy, sleep, eating behavior, growth, puberty, bone quality of children and quality of life of parents. She and her team also conducted a study on the effect of behavioral treatment of sleep problems in children. Finally, she described which new tests for neurocognitive functioning and growth are and are not feasible in children with AS. She especially thanked all children with AS and their parents for their trust in the ENCORE Expertise Center and sharing their knowledge and experience!
You can access her dissertation at Angelman Syndrome in Children (ogc.nl). If you are interested in a paper version of the dissertation, please contact us at angelman@erasmusmc.nl
It was a great day for the ENCORE Expertise Center for AS!